
In the early hours of a sparkling June morning, I walked into Cook Children’s Medical Center in Fort Worth, Texas, a nationally ranked network of clinics catering to all manner of childhood illnesses. The doors had just opened, and the first patients were trickling in alongside me.
I had come to meet Dr. Angel Hernandez, the director of the hospital’s pediatric epilepsy program. A trail of wall-mounted signs led me to the pediatric neurology ward, a bright and airy space with flat-screen TVs running cartoons nonstop. Decorative kites were strung up in the corridors, and rainbow curtains lined the windows. Some of the kids in the waiting area that morning were alert and awake, others groggy. Some were strapped into special strollers designed for children with mobility problems, and some had shaven heads and healing scars. Hernandez came out to greet me, and I was surprised he recognized me after what felt like a very long time. He had diagnosed me with epilepsy in 2004 and treated me for several years.
For most people with epilepsy, diagnosis sets off a gauntlet of trial-and-error attempts to find the right medication. The process is tortuous, with seizures alternately dying down and flaring up while side effects— fatigue, nausea, liver damage, and more—develop without warning. This is partly due to the fact that “epilepsy” is actually a broad category that includes a number of distinct seizure disorders. About 30 medications approved by the U.S. Food and Drug Administration are currently used to treat these conditions, and when a person first begins having seizures, there is often much tinkering with combinations and dosages. I spent years battling side effects like vomiting, dizziness, drowsiness, and severe headaches, which were alleviated only by yet another prescription medication. Parents who endure enough sleepless nights caring for a sick child can become desperate for a cure.
In the past few years, just such a cure has seemingly presented itself. Amid the less common remedies that can be found on the internet—special diets, meditation, biofeedback, surgical implants—a new product has recently gained prominence: CBD oil (sometimes known simply as “hemp oil”), so named for its chief chemical compound, cannabidiol, which occurs naturally in cannabis plants. In online forums and news articles, CBD has been hailed as a new frontier in epilepsy treatment, with parents testifying that it managed to stop their children’s seizures when nothing else could.
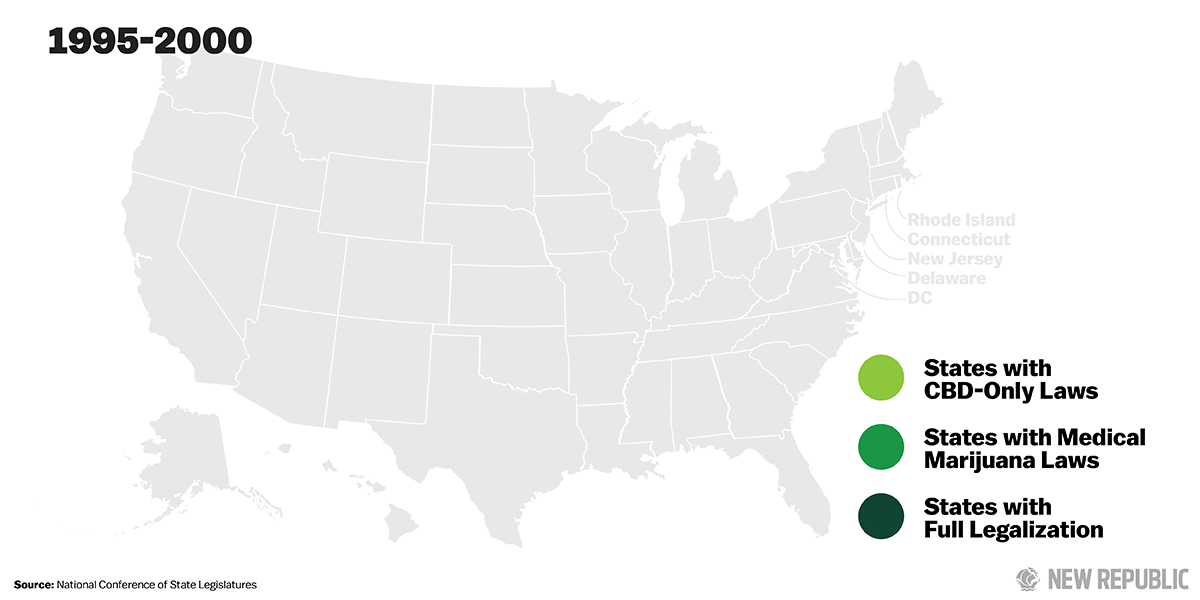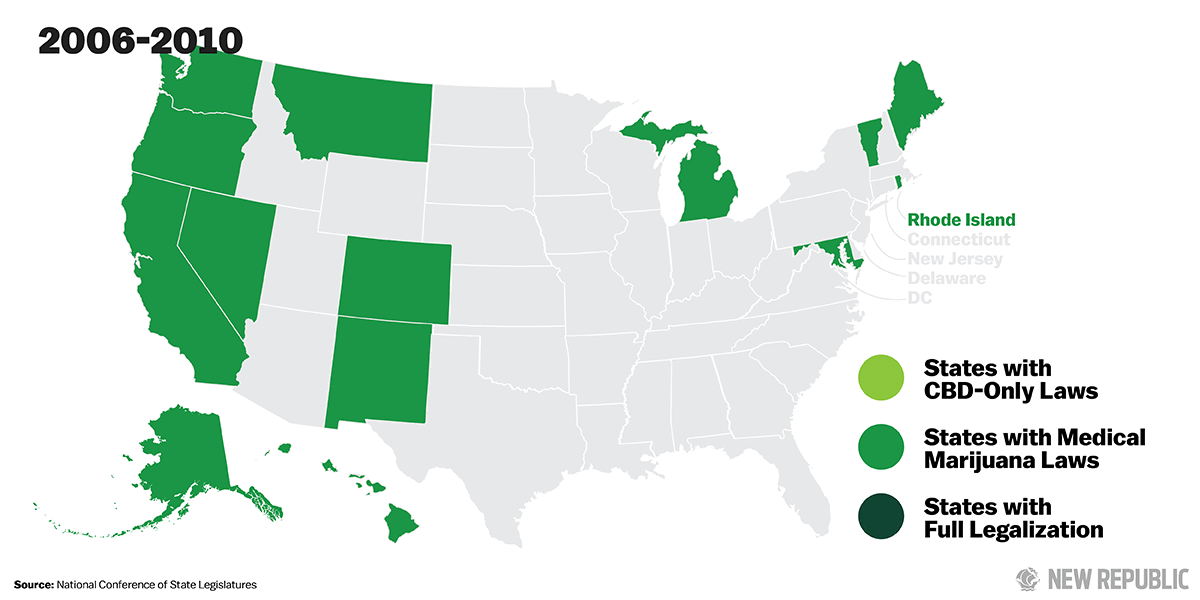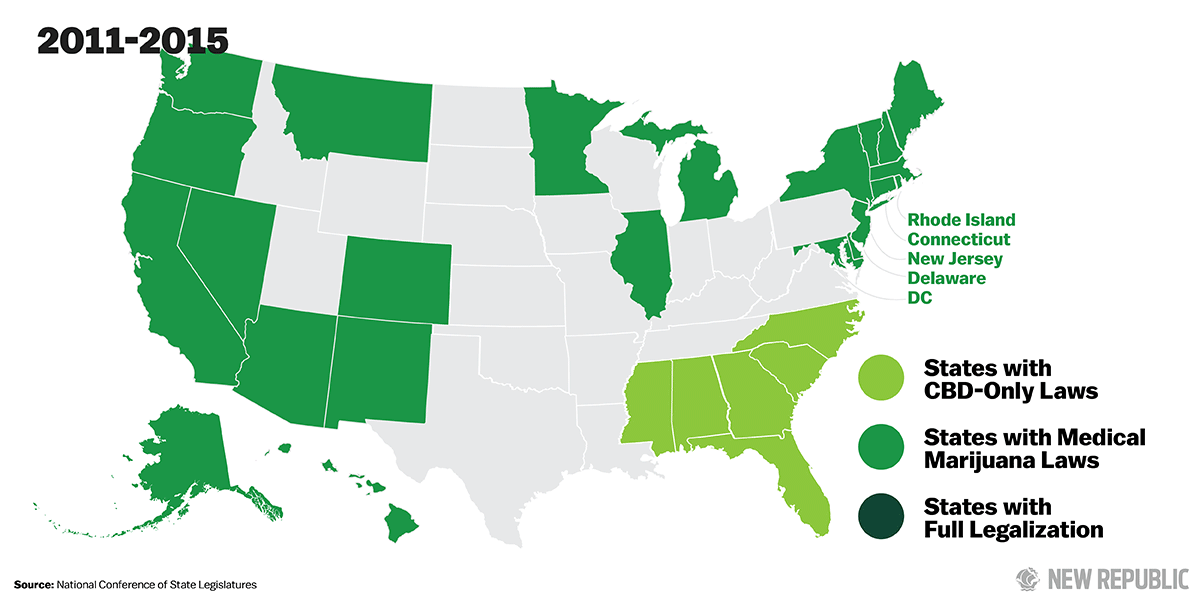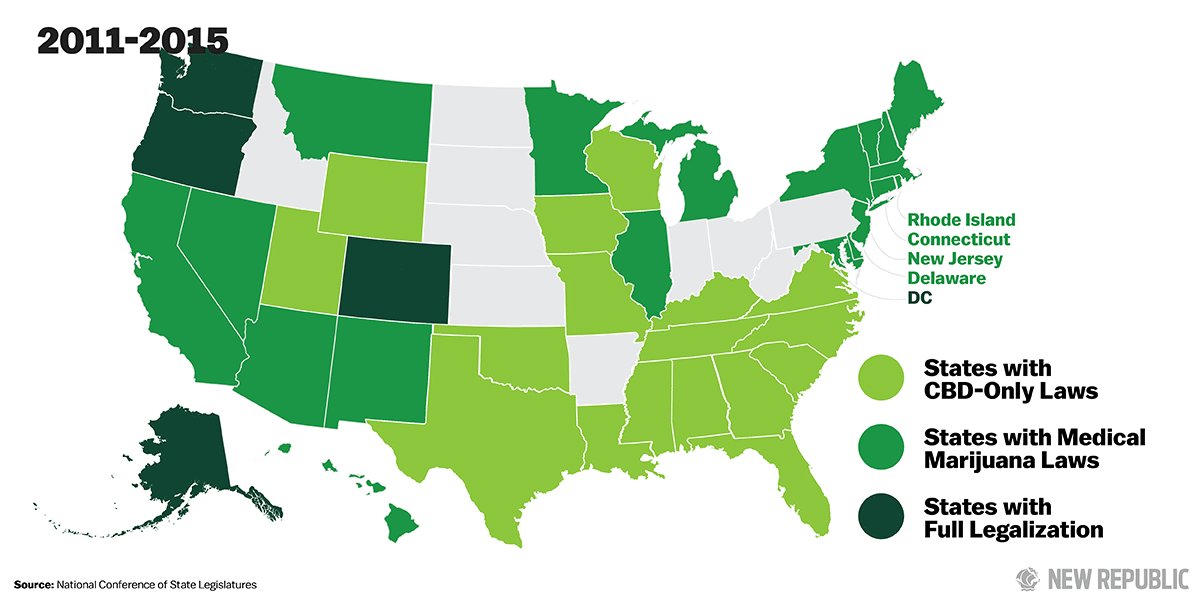
Over the past two years, 17 states have passed laws legalizing CBD so that patients can obtain the drug without fear of prosecution from local authorities. For intractable childhood epilepsies—the sorts of seizure disorders that for centuries have ruined lives and shattered families, the ones even specialists like Hernandez dread—CBD could be a miracle cure.
In his office, however, Hernandez was wary of the CBD boom. He advises well-meaning parents to think twice about voyaging into the world of over-the-counter hemp oil treatments, even if their circumstances are dire. “It’s a huge gimmick that a lot of companies are using,” Hernandez said. “You don’t know what you’re getting. ... There’s a major quality problem.”
Indeed, hemp oil products have grown out of a market largely devoid of regulations or safety protocols. The state of the CBD industry harks back to the age of elixirs and potions hawked from covered wagons to the awed denizens of pioneer towns. There are no industrywide standards in place to ensure that CBD oils are consistently formulated batch-to-batch. There is no regulatory body screening products for pesticides, heavy metals, solvent residues, and other dangerous contaminants. The laboratories that companies contract to test their CBD products are themselves neither standardized nor consistently regulated. No medical research exists to recommend how much CBD a patient should take, nor is there detailed, reliable documentation of how CBD interacts with most epilepsy medications.
Taking CBD oil is like drinking milk and calling it calcium, Hernandez said: There’s some in there, but at very low concentrations dispersed among a host of other ingredients. And what those other ingredients are is anyone’s guess. “The thing to know is that CBD hasn’t gone through the safety controls, the efficacy controls that we usually use, the clinical trials,” Hernandez said. “The jury is still out regarding how safe this drug is.”
In the absence of regulation and research, the CBD industry trafficks in hard-sell marketing and a gray haze of half-truths, all the while profiting off the hopes of the desperate.
Hemp oil has never been as popular as other marijuana products. With little to no THC, CBD-rich strains of cannabis don’t deliver the pleasant buzz recreational users seek out in marijuana. In the 1970s, however, scientists found that cannabidiol was effective in reducing seizures. The brain’s endocannabinoid system contains receptors that respond to CBD, producing anticonvulsant effects. Being plant-derived and native to the brain’s own chemistry, CBD is therefore one of the most natural options for seizure treatment available today. Still, not many people took interest in CBD until 2013, when a CNN documentary special, Weed, hosted by the network’s chief medical correspondent, Dr. Sanjay Gupta, highlighted CBD’s effectiveness in combating seizures. Since then, demand for hemp oil products has exploded.
CBD’s potential usefulness in treating certain conditions is yet another argument in favor of legalizing the entire cannabis plant. Removing cannabis from the federal list of Schedule I narcotics that are illegal under the Controlled Substances Act would allow scientists to research its full medical potential and pharmaceutical companies in the United States to develop marijuana-based drugs and submit them for FDA approval. Government-regulated labs could test products like CBD oil to ensure safety and quality. Doctors could prescribe marijuana- based medicines with full knowledge of potential side effects and drug interactions, and without fear of losing their medical licenses or being thrown in jail.
Support for legalization has steadily grown over the last several years. Today, medical marijuana is legal in 23 states and the District of Columbia. And even federal officials have begun to soften their stances. Last fall, outgoing Attorney General Eric Holder signaled his support for removing marijuana from the list of Schedule I narcotics. “I think it’s certainly a question we need to ask ourselves, whether or not marijuana is as serious of a drug as heroin,” Holder said. This summer, Chuck Rosenberg, the acting administrator of the U.S. Drug Enforcement Administration, acknowledged that marijuana is not as dangerous as other Schedule I drugs and announced his agents would not be prioritizing marijuana enforcement. Still, as long as marijuana remains illegal under federal law, the haphazard system in which it is studied, produced, and distributed will remain, and Americans will not be able to take full advantage of its medicinal properties.

Today, dozens of companies produce CBD in an array of forms. CBD can be inhaled through vape pens, applied in topical salves, ingested in edibles, or swallowed in oil-based tinctures. Oil has become the dominant CBD delivery method for kids with epilepsy, since it is easy to administer and ingest, and there is no shortage of it available for sale online. There are dozens of companies boasting names like Healthy Hemp Oil, Dose of Nature, and Natural Organic Solutions, each of them selling CBD products at prices ranging from trivial to dizzyingly steep. You don’t have to look hard to find them. If you have a PayPal account and $100 to spare, you could have a vial of hemp oil delivered to your doorstep.
One of the biggest players in this new industry is Medical Marijuana, Inc., a company formed in 2009 that operates out of Poway, California, just north of San Diego. It has played a leading role in the so-called Green Rush, as businesses have moved quickly to capitalize on the gradual legalization of marijuana for medical and recreational purposes by states across the country. The company’s spokesman, Andrew Hard, boasted that Medical Marijuana, Inc., “created the CBD industry and was first to market with CBD products.” Through its various subsidiaries, Medical Marijuana, Inc. sells some of the most recognizable products on the cannabis market— everything from Cibaderm CBD-infused shampoo to CanChew chewing gum. In 2014, the company generated $14.5 million in revenue.
Among the company’s many offerings is Real Scientific Hemp Oil, which it sells through its subsidiary HempMedsPx, also based in Poway. On its web site, HempMedsPx describes how its hemp “is grown in northern European microclimates, without the use of any pesticides, herbicides or chemical fertilizers.” The company promises that it “continuously scrutinizes and improves the processes to meet all regulations and exceeds quality standards.”
When Brandon Krenzler’s daughter Mykayla was diagnosed with a form of childhood leukemia in 2012 at the age of seven, he began researching medical marijuana products that might ease her symptoms and blogging about the results. The next year, he received some samples of Real Scientific Hemp Oil, which he administered to Mykayla. But the oil made her sick.
Concerned about Mykayla’s stomach cramps, Krenzler, who lives in Portland, Oregon, sent a sample of the oil off to Going Green Labs in Albany, Oregon. Like most labs catering to the cannabis industry, Going Green mainly performs THC potency tests. According to Krenzler, when the lab tested his sample, it found that the Real Scientific Hemp Oil contained much more THC than HempMedsPx had claimed—3.8 percent, instead of roughly 1 percent. Krenzler said he was “disturbed” by the finding, and also by the implications it had for other parents of sick children. Medical marijuana is legal in Oregon, but Krenzler noted that in other states that have not legalized pot, anyone purchasing a product with more than a trace amount of THC could find themselves in legal jeopardy. “I feel that HempMeds had misrepresented their product,” Krenzler said.
Elias Anderson, one of the owners of Going Green, said representatives from HempMedsPx approached him after Krenzler published the lab’s findings on his blog. “They were like, ‘What are we gonna do about it?’” Anderson recalled, “And I was like, ‘Nothing. We have standards, and I stand behind my test results.’” Still, the company’s representatives were insistent and advised Anderson to have Kenzler take down the lab’s findings. In an email to the New Republic, Hard, the Medical Marijuana, Inc. spokesman, contended that the sample of hemp oil that Going Green Labs tested had been “tampered with” by a competitor after Krenzler obtained it. “HempMedsPX, if anything, told the lab they cannot publish results from products [for which] they had no chain of custody tracked,” Hard said, “and if they did—that could prove to be very bad for the lab.” He also characterized Krenzler and Anderson as “haters” of Medical Marijuana, Inc., and suggested that much of the criticism of the company and its products comes from commercial competitors.
Yet when one looks at the industry more broadly, there is cause for concern. In February, as part of an investigation into the marketing claims of six hemp oil companies, the FDA analyzed 18 CBD products. What it found was disturbing: Many of these supposed CBD products were entirely lacking in CBD. Of the products tested, six contained no cannabinoids whatsoever. Another 11 contained less than 1 percent CBD. The product that tested highest in CBD, at 2.6 percent, was a capsule for dogs. In states that have legalized CBD, regulations can require CBD products to contain at least 5 percent CBD, more often 10 or 15 percent.
Low concentrations of CBD aren’t the only concern, either. Cannabis plants are hardy and tough, and their thick stalks possess a special property: bioremediation. When grown in contaminated soil, hemp plants absorb heavy metals and other chemical waste, effectively cleansing the terrain. While all plants absorb some chemicals from the soil, the structure, size, and genetic makeup of hemp make it especially adept at this task. Cannabis is so effective that crops of industrial hemp were planted in the aftermath of the 1986 Chernobyl disaster to help purify heavily irradiated soil. When hemp stalks are used for fiber, paper, and other nonconsumptive industrial purposes, the contaminants absorbed into the plants pose no threat to humans.

Ingesting industrial hemp is another story. A 2001 study conducted in a British hemp processing plant found that residual industrial hemp dust contained microorganisms and other contaminants with the “potential to cause a range of ill health problems” if inhaled.
There are ways to strain dangerous contaminants out of raw hemp paste. And most companies stand behind their quality and safety procedures. “We continuously test all our products ... to ensure our consumers get the levels of natural constituents they expect from the quality hemp stalk oil they purchase,” HempMedsPx states on its web site. “Additionally, all our products are tested for safety, to ensure there are no solvents, heavy metals, or other potentially harmful materials in our oil. Because we take these steps, we are always confident in our products, and you can be too.”
Stuart Titus, Medical Marijuana, Inc.’s CEO, argues that hemp plants have a natural ability to rid themselves of contaminants. “The natural capabilities of the plant break down some of these long, large-chain molecules to their base components and, over time, just render them much less toxic,” he told me.
Dr. Ethan Russo, medical director at Phytecs, a biotechnology company spearheading research into plant- based medicines and the endocannabinoid system, took issue with Titus’s claim, however. “Bioaccumulators can recruit heavy metals from the soil,” Russo said, “but breaking them down would be alchemy.” Government regulation of the pharmaceutical industry is designed to protect consumers from unfounded scientific claims.
In the end, companies like HempMedsPx are asking consumers simply to trust them. CBD oils are never subjected to systematic testing by any U.S. regulatory body. The FDA regulates all pharmaceutical labs in the country. But cannabis labs like the ones that HempMedsPx and others use are not, because cannabis is not federally recognized as a legal drug.
All of this makes CBD remarkably difficult for even the most dedicated health care providers to manage safely. Dr. Kelly Knupp, an associate professor of pediatrics and neurology at the University of Colorado, and the director of the Dravet Syndrome program at Children’s Hospital Colorado, said families of epileptic children have tried to bring CBD oils to the hospital for testing. “They’re just concerned that they don’t know exactly who’s growing [the hemp],” Knupp said. “They know it’s not being regulated.” But because CBD is a Schedule I controlled substance, high-tech, regulated laboratories, like those at the University of Colorado, can’t accept, store, or test CBD oils, lest they risk prosecution. “There is no such lab that can take that product,” Knupp said, which leaves any testing up to the unregulated testing centers that cater to the cannabis industry.
Despite the fact that marijuana remains illegal at the federal level, companies like HempMedsPx claim their CBD products are legal in all 50 states. According to a legal opinion written by Medical Marijuana, Inc.’s attorney and submitted to the New Republic, “HempMedsPx’s CBD hemp oil, containing naturally occurring CBD and miniscule amount of THC, is exempted from the definition of marijuana, is not a controlled substance, complies with the Controlled Substances Act, and is legal on the federal level.” The opinion is based in large part on a 2004 court ruling which allowed the importation of hemp food products derived from the mature stalks of cannabis plants.
Yet the DEA has stated unequivocally that it considers CBD to be illegal under the Controlled Substances Act. “CBD derived from the cannabis plant is controlled under Schedule I of the CSA because it is a naturally occurring constituent of marijuana,” Joseph Rannazzisi, the deputy assistant administrator of the DEA, told a congressional panel in June. “While there is ongoing research into a potential medical use of CBD, at this time, CBD has no currently accepted medical use in the USA.” Moreover, DEA spokesman Eduardo Chavez told the New Republic that Medical Marijuana, Inc.’s in-house opinion with regards to CBD has no merit. “The bottom line,” Chavez said, “is the oil is part of the marijuana plant, and the marijuana plant is currently a Schedule I controlled substance under federal law.”
The DEA isn’t the only government agency scrutinizing CBD vendors. To fend off the FDA, hemp oil companies contend their wares are not drugs but “dietary supplements.” Despite the suggestive “meds” in the company’s name, HempMedsPx is careful to note on its web site, “Although some of our founders are medical professionals, we cannot make medical claims about the benefits of our products.” Others are not quite so nuanced in their marketing. The internet is flooded with CBD products claiming to treat everything from seizures to arthritis to skin conditions and other maladies.
For example, the six hemp oil companies the FDA had investigated in February had explicitly advertised CBD products for use in the “cure, mitigation, treatment, or prevention of diseases.” The agency sent warning letters to the companies, ordering them to change their product labeling or face potential legal action. Then, in May, the FDA announced it was excluding products containing cannabidiol from its definition of dietary supplements altogether. Hard, the spokesman for Medical Marijuana, Inc., said the company views “these developments as positive because this allows the debate regarding CBD to come to the forefront.” He characterized the FDA’s May announcement as “an opinion” and added, “Medical Marijuana, Inc. and HempMeds, along with industry associations, are working on determining how we can come to a mutual understanding on the matter with the FDA.”
Warning letters to CBD companies nonetheless litter the FDA’s Health Fraud list, but cracking down on online purveyors is like a game of whack-a-mole, and CBD products remain widely available.
Of course, parents who desperately want to find something—anything—that will help their sick children, don’t have the luxury of caring whether CBD is classified as a drug or a supplement, or whether they get it from a doctor or an online retailer. One reason why people are willing to trust companies like HempMedsPx is that, for some, CBD oil does seem to work.
In early June, I met with Penny Pennington Howard, a mother of three, who lives in Carrollton, Texas, about 25 minutes outside of Dallas. Posted in the glass of her front door are two signs you can’t quite make out from the sidewalk: one asking visitors not to smoke, as oxygen treatments are in use; the other a yellow diamond informing guests this is the home of a special needs child. Penny welcomed me inside, out of the glare of the sun, and led me through her living room into her kitchen, where her kids were gathered for lunch. Seth, then eight months old, was plucking cereal off the tray of his highchair, while Lily, seven, was darting back and forth between the countertop and table. Harper, a blond five-year-old with hot pink toenails, was reclining in her “tomato chair,” a molded plastic seat with straps to help keep her steady.
Harper was diagnosed as an infant with CDKL5, a rare genetic condition doctors only discovered in 2004 and that afflicts roughly 600 people worldwide. The disorder shares its name with the minute particle of DNA it affects, a gene responsible for the production of a protein crucial for neurological development. Symptoms of CDKL5 include intellectual disability, developmental delays, breathing and vision problems, limited or absent speech, poor muscle tone, and, perhaps worst of all, frequent seizures.

It was the seizures that tipped Penny off that something wasn’t right with Harper after she and her husband Dustin brought her home from the hospital as a newborn. Several months later, having tried a battery of epilepsy medications and still without a diagnosis, Penny and Dustin flew to Boston with Harper to see an expert in infant seizures. It was there they first heard of CDKL5. “This is the point where life changed significantly,” Penny said, “because now we had this diagnosis. You know, this abnormality in our family that we cannot fix.”
No medication seemed to provide a great deal of relief for Harper’s symptoms. But in 2013, three years after their trip to Boston, Penny and Dustin caught an installment of CNN’s medical marijuana documentary and began researching what they could obtain in Texas, where medical marijuana is illegal. Their internet searches soon led them to HempMedsPx and Real Scientific Hemp Oil. The company sent Penny a vial of hemp oil, which she administered to Harper that September.
“The week before we tried it, we had 64 seizures,” Penny told me, noting those were only the visible seizures, while unseen neurological events would likely push the number into the hundreds. “We administered hemp oil, and the next week we logged in 28 seizures. ... The very next week, her second week on the hemp oil, we logged none.” Penny paused and repeated herself, as though she could still only half believe the miracle: “None.”
But all was not well. Harper has continued to experience health issues related to her condition. And seven months after starting to use CBD oil, Harper’s seizures returned— although not as frequently as before. Penny uses eleven iPhone reminders to keep track of Harper’s daily regimen of medications and food, and she records all of Harper’s seizures in a thickly bound black book. But as her parents continue to closely monitor Harper’s health and adjust her medications accordingly, her doctors are tightly limited in the advice they can offer when it comes to CBD oil. “There’s no research on this product, so they don’t say it’s good or bad. They just say, ‘Don’t stop giving it,’” Penny told me.
Hernandez said interactions between FDA-approved pharmaceuticals and CBD oils are a serious concern. “What we’ve found so far is that [CBD] can actually affect the levels of some of your epilepsy medications,” Hernandez told me. The diarrhea and vomiting associated with CBD oil ingestion can lower the levels of other drugs in patients’ bloodstreams, while the way the body absorbs CBD can raise the levels of certain medications.
For kids with severe forms of epilepsy, changes in medication levels can be extremely dangerous. “If their levels go low, they’re at increased risk of seizures, which could lead to an emergency room visit or an ICU stay,” Knupp said. “On the other hand, if their levels go high, their side effects can increase dramatically.” Side effects from epilepsy medications can range anywhere from drowsiness to vomiting to heart arrhythmia, Knupp noted. “For some people that could mean a minor inconvenience, but for some patients it could be life-threatening.”
Knupp said she has seen children in critical condition after families decided to switch from pharmaceuticals to CBD products. “We had a couple of those children ... who landed in our emergency room or in our ICU because of escalation of seizures that was likely more due to stopping standard anticonvulsants,” Knupp said.
Neurologists are skilled at predicting side effects and interactions between well-researched pharmaceuticals. But due to the dearth of reliable research about CBD, doctors like Hernandez and Knupp cannot guide their patients in its use. If there are adverse reactions, Penny will find out because Harper will suffer through them. She has had to figure out through trial and error how best to mix and measure Harper’s oils. The bottom line, Penny said, is simple: “We are the research.”
To this point, CBD oil has existed in a kind of liminal space— at once an illegal drug, a legal medication, and some kind of “dietary” supplement. It’s possible this could change in the coming years, however. GW Pharmaceuticals, a U.K.-based firm, has developed a “pure CBD” medication called Epidiolex that has shown promising test results. It is currently on a fast-track to receive FDA clearance. For some patients, Epidiolex could be a miracle cure. This summer, in Wired magazine, writer Fred Vogelstein chronicled his family’s own struggles to find an effective treatment for his son’s epilepsy—including experiments with hemp oil— and the immense hurdles they overcame to gain access to Epidiolex prior to its FDA approval. The drug could be for sale on pharmacy shelves in the near future, though exactly how near is hard to say.

The arrival of Epidiolex is unlikely to erase the unregulated CBD market, however. For one, Epidiolex has been studied only in connection with a small number of epileptic conditions. If and when Epidiolex makes its way to drug stores, it will be approved only for the treatment of Dravet Syndrome and Lennox-Gastaut Syndrome, two rare forms of catastrophic epilepsy. People like me, with comparatively mild Janz Syndrome, and people like Harper, with extremely rare conditions like CDKL5, may still be out of luck.
As the demand for CBD products has increased, some states have started to take action. Over the past two years, 17 states have passed “CBD-only” laws, assuring parents who purchase CBD oil to treat their sick children that they won’t face arrest or prosecution from state law enforcement for possessing what the federal government still considers a Schedule I narcotic.
These CBD-only laws also attempt to impose some regulation on CBD oils, such as establishing how much CBD and THC such products must contain. For example, on June 1, the day I sat down with Hernandez in Fort Worth, Texas, Governor Greg Abbott signed the state’s Compassionate Use Act into law in Austin. The law requires that all CBD products contain no more than 0.5 percent THC and at least 10 percent CBD. However, the bill does not specify how the state plans to enforce this requirement. The law contains no language outlining how laboratories can test CBD products, what kinds of standards they would use, or who would regulate them.
Out of the 17 states that have passed CBD-only laws, five— Missouri, Florida, Mississippi, Louisiana, and Texas—would also establish licensed cultivation centers to grow high-CBD strains of cannabis, which could be turned into oils and other CBD products. This would cut down on the demand for CBD oil from unregulated manufacturers abroad. Even then, though, impediments remain. In Missouri, for example, two neurologists recently refused to prescribe CBD oil for an eight- year-old boy suffering from seizures, citing concerns over federal law and the safety of non-FDA approved products.
On the federal level, several bills currently before Congress seek to change the way the government treats CBD. One such bill, the Compassionate Access Act, would exclude CBD from the classification of “marijuana” and remove both from the DEA’s list of Schedule I controlled substances. Rescheduling CBD in such a way would make research and cultivation of CBD much easier.
Even without changes at the federal level, there are steps that states could take on their own to make the CBD market safer. States with broad marijuana legality or CBD-only measures could mandate the calibration and regulation of testing labs, and use them to conduct safety testing. They could fund research into the benefits, dosing, and drug interactions of CBD through their public university systems. Medical boards could redouble efforts to educate physicians in what research exists regarding medical marijuana in all its incarnations, so that doctors are prepared to prescribe and manage these medications as they become available.
Of course, the easiest solution, advocates say, is for the federal government to legalize cannabis completely. If cannabis were legalized—the whole plant and all its extracts, no confusing singling-out of specific compounds or anatomical features—then U.S. drug companies would be able to carefully cultivate and research its medicinal properties, and submit their findings to regulatory bodies like the FDA for trials and approval.
For patients suffering from seizures, the legalization of cannabis would be a decisive turning point. Epilepsy makes you desperate. Seizures are painful, sometimes debilitating. And then there are the aftershocks: broken teeth, bruises and cuts, lost time, humiliation. People with epilepsy are often depressed, and have more than double the suicide rate of the population at large. Epilepsy is also associated with a syndrome known as Sudden Unexpected Death in Epilepsy, wherein a previously healthy person with epilepsy simply dies without warning or explanation. Grinding on without relief isn’t an option, but getting help is enormously expensive. Research conducted by Charles Begley, a professor of public health at the University of Texas, found that epilepsy treatment costs between $8,500 and $11,000 per year. Real Scientific Hemp Oil is no less expensive than its pharmaceutical counterparts, with no assistance from insurance. A single three-gram vial costs $149, while a six-pack of 10-gram tubes can cost $1,999 (or $1,599 on sale). HempMedsPx suggests a “serving size” of 0.5 ml twice daily. Only when these drugs are recognized as such will insurance pick up the tab.
During my visit, Penny showed me how she administers Harper’s CBD oils. We stood in her kitchen, where a window opened onto a vista of green grass and a wooden swing set out back. After carefully mixing and measuring Harper’s oils, Penny poured the liquid into a jumbo-sized plastic syringe. “We put this all online,” she told me, referring to the several YouTube videos she has made to help other parents administer hemp oil. Penny leaned down over her daughter to fit the tip of the syringe into her gastronomy tube, and I stood by silently. Harper looked at Penny, and Penny smiled back at her, and eased the plunger down.