I learned of my BRCA mutation on a Thursday evening, the day after my grandpa became ill with possible pneumonia. It was clearly the end, so my parents went down to Tennessee that weekend to sit with the other relatives and watch Roscoe, who was Gov to his grandkids, die at home in his bed. I had been invited, of course, but I couldn’t go—I didn’t think I could bear to watch this up close while still reeling emotionally from the news that I carried the same cancer-causing BRCA1 genetic mutation as my mother. Over the weekend, Gov took a turn for the worse. And so, the following Wednesday morning, I got the call. “He’s gone,” my stoic father said, voice cracking. I’d only ever seen my father cry once, when we watched Goodbye, Mr. Chips as a family in my teens, though my mother swears he cried at my birth too.
That afternoon, out of respect for my grandpa, I put on all black, not knowing that it marked the beginning of a fashion era for me, and jumped into the car to head to my mammogram. I had thought about cancelling but decided against it; I was simply too nervous about my BRCA diagnosis, which had arrived on the phone a week ago. The fact that I had inherited a harmful BRCA1 mutation from my mother spiked my risk of breast cancer from the normal 12 percent lifetime chance to at least 55 to 65 percent, maybe higher, and raised my ovarian cancer risk from the normal 1.4 percent lifetime chance to at least 39 percent. In addition, BRCA1 related breast cancers tended to strike younger and more aggressively than cancers in the normal population. In addition to my mother, breast and ovarian cancer had ravaged my family tree—afflicting my grandmother three times and killing both of her sisters.

This would be my first mammogram in several years. Of course, my grief added to my already-fraught emotions about the procedure, and today, nothing would go well for me. For starters, the woman at the front desk explained to me that they didn’t do mammograms on women as young as I was then—27. “But I have the gene,” I managed to force out. “My mother got cancer at 30.” If the women in my family had waited until the recommended screening age of 40, they’d all be dead. “No exceptions,” the receptionist said. She noted that my new insurance company hadn’t approved the procedure and pointed me to the phone in the next room. I teetered on the verge of hysteria, could feel myself protesting ever more shrilly, could feel my eyes well up. I would call the insurance company in the next room. I rifled through my purse for my insurance card only to discover that in my wrought emotional state, I’d left the house without my wallet. Of course. Even if I could somehow work this out without my insurance card, I couldn’t pay my part of the bill. I stalked back to the car and scrounged in the cushions for enough money to pay for parking—in my haste to leave the office, I hadn’t validated my ticket.
One might imagine a treatment center geared to people like me, where I could simply go in every six months for screening and get all the tests at once. It would be like some sort of rational paradise where I only had to spend two hours a year on this genetic condition. Instead, I had to patch together my own treatment, managed largely by institutions that were not accustomed to BRCA patients and their surveillance needs. I’d even heard horror stories from other women about doctors who weren’t as informed about BRCA as they should have been, doctors who told them that a family history of cancer “didn’t count” if it was on their father’s side, or who were not up to date on the surveillance protocol.

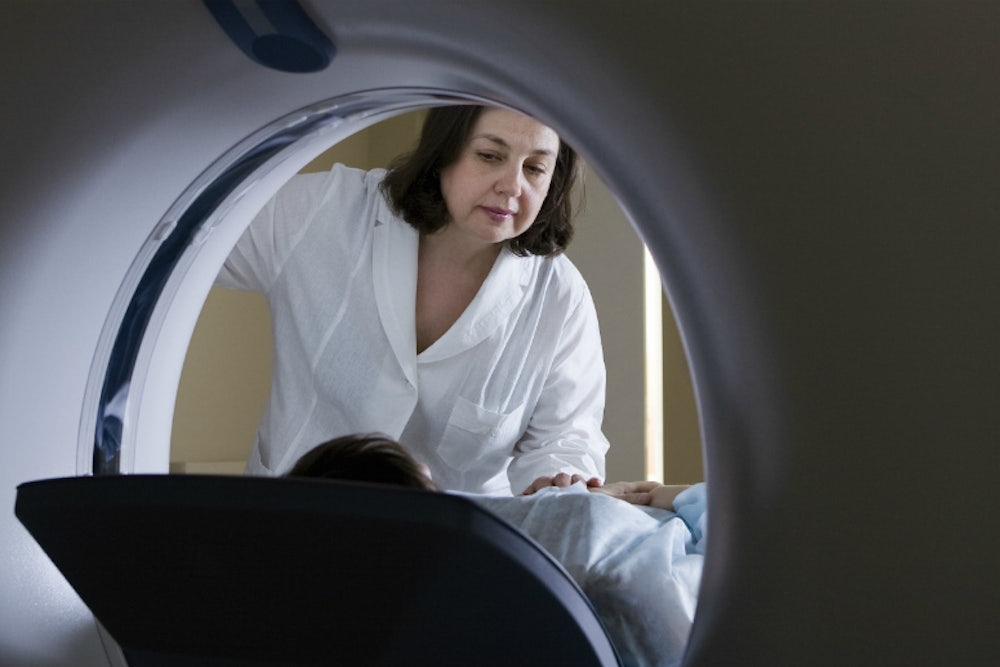
And by the way, BRCA women are supposed to have a whole lot of surveillance. It involves a battalion of doctors—by the time I’d been through it once, it seemed like I’d been felt up and fingered by every clinician in the state. According to the National Comprehensive Cancer Network (NCCN), an alliance of 25 of the world’s leading cancer centers that publishes standard of care guidelines for many sorts of cancer-related treatment, among them, surveillance for BRCA patients, if you want to keep both your breasts and ovaries, then you must scan the ever-loving snot out of them, though to be fair, not even medical professionals have much enthusiasm for ovarian screening. Once or twice a year, a professional should feel you up for lumps. Once a year, your breasts get squeezed tightly between two plates while radiation is shot through them to make a picture—a mammogram. Because many BRCA patients receive screening while they have young, dense breasts that are hard to read via mammography, and because MRIs are a more sensitive scan, you’re supposed to alternate mammograms with MRIs. Preferably, each MRI happens on certain days of your cycle and involves having dye pumped into your veins while you lie prone in a giant tunnel with all your body jewelry taken off while the loud clicks of a magnet get pulsed through you. As it turns out, I am one of the unlucky few with an allergy to the contrast dye used, which means I have to take steroids on the day of, or occasionally a pretty intense dose of Benadryl, which addles me.
That sounds like two trips to the doctor: one for a mammogram and one for an MRI, right? Wrong. It’s four—two trips to the oncologist who feels me up and orders the tests, and then two to the lab to have different sorts of beams shot into my breasts. Of course, if they find any “suspicious” areas—and the more you scan your breasts the more likely this is—then you have a breast ultrasound, where they squeeze jelly over your boobs and glide a wand over them, using sound waves to look inside. Sometimes this happens at the same time as your other appointments. Worst case scenario is that you have to make a new one.
According to oncologist Dr. Otis Brawley, chief medical and scientific officer of the national health organization the American Cancer Society, part of the reason for this surfeit of appointments lies in how doctors get paid. “I treat patients with cancer,” he tells me, “and this is actually a national shame. We see the patient on Monday, [and] write their chemo orders to be administered on Tuesday, because if the chemo is administered the same day we see the patient and write the chemo, we get paid less.” Similarly, he says, if I were to go to a high-risk clinic and see multiple doctors on the same day, my insurance would pay as if it had been a single visit—all the doctors would be splitting the reimbursement. It’s not efficient, and it’s certainly inconvenient for patients, but it’s the way the system currently works. My rational paradise does not exist in part due to bureaucratic red tape.
If they do find something inside your breasts during an exam, often they don’t tell you at the appointment either. They’ll simply say they want to look a little closer and then get back to you days or weeks later. During that time, you wonder whether you screwed up on this round of Russian roulette—the ten-bullet gun has as few as four or as many as eight or nine rounds in it, after all—and whether this time there will be something in the chamber when you pull the trigger.
If breast cancer surveillance in BRCA patients is an exercise in anxiety, then ovarian cancer surveillance is an exercise in futility. Doctors have limited tools—a physical pelvic exam, a transvaginal ultrasound, and a blood test—that have not been proven effective in catching ovarian cancer or in preventing death from the disease. Still, like many other BRCA patients, I go through the motions; surely doing something is better than doing nothing. Am I crazy for using an imperfect test to screen for a cancer that is rare in the general population? Or is the medical profession gaslighting me? Am I really a hypochondriac, or do this doctor and this technician just not understand the risk I face, why I want my blood drawn even though the substance they test for is only produced by certain types of ovarian cancer and can fluctuate for other reasons, like stress or your period?
NCCN recommends getting into a study on ovarian surveillance in high-risk women, because there is not enough data on this, but my gynecologist is focused on delivering babies, not on catering to whiny women like me. Since my ovarian surveillance comes through her, though, that’s another three to four appointments a year—for some reason I can’t have my ultrasound during my yearly Pap smear and have to come back for another test. We argue about blood draws too—she thinks they’re useless, while according to my research, the efficacy of doing these every six months for high-risk patients has not been evaluated yet. Sometimes I’ve used up all my arguing points on the biennial ultrasound though, so I succumb to her wishes and skip it. This is one of the horrors of BRCA—the fear that you might know more about BRCA than your doctor and not get the care you need if you follow her advice, balanced against the fear that you’re paying her for her expertise, so she probably knows more than you do, and that you might be acting like a crazy, high-maintenance bitch. This is why specialists get paid the big bucks.
Altogether, that’s a minimum of seven trips to the doctor each year for screening—sometimes more if you need an ultrasound or a biopsy. This is in addition to any standard maintenance, such as going to the dentist or for a physical or to see specialists like an eye doctor, allergist, and so on. I don’t like to spread them out—I try to do them all in one or two weeks. But it is a lot of doctors’ visits, accompanied by uncertainty and anxiety around the test results. Watchful waiting has its price. The question is whether it’s worth that price, and when I looked into the issue, I found that the answer is surprisingly complex.
Detection tests for cancer walk a thin line. Ideally, such tests would catch all cases of cancer—they wouldn’t return positive results for things that looked like cancer but weren’t (false positives leading to overdiagnosis), but they also wouldn’t miss any cases of cancer (false negatives leading to underdiagnosis). And while we’re wishing for an ideal world, perhaps we could have one test that found all cancers and then a second test to tell you which cancers were lethal and which ones weren’t, Brawley suggests. A perfect detection test would be, in the words of Goldilocks, “just right.” But since we don’t live in a fairy tale, in the real world, over- and underdiagnosis are balanced against one another. You can think of cancer screening like a net strung across a river, fishing for salmon. If I weave it tight enough to catch all the salmon that come downstream, chances are good it’ll also catch a bunch of not-salmon—turtles, otter, river weed, stuff that I’m not interested in. On the other hand, if I weave the net loosely enough to let the otter and turtles pass, some of the smaller salmon will swim through—I won’t catch them all. Use a very sensitive test, and you’ll get lots of false positives. Use a not-sensitive test, and you’ll get false negatives. It’s pretty hard to win the game.
Surveillance rests on the idea that there is a long march from normal cells to atypical cells to cancerous cells and finally to tumors and metastasis. The reasoning goes that if it’s found when it’s baby cancer—cute and cuddly—but before it becomes Voltron, it’s easier to kill. This idea of carcinogenesis, that cancer starts as normal cells which slowly become atypical and then malignant, suggests that catching cancer earlier in its development, when it is not cancer but “pre-cancer,” can help prevent cancer death. But not all cancer is suited to these methods. It’s a bit like cooking onions—if you put them over high heat, you have to watch them carefully to make sure they don’t burn. But if you put them on low, then they take a longer time to go from raw to burnt, and you don’t have to be so attentive. Let’s say I’m predestined to get cancer. If my cancer is aggressive, the cells in my body might change from normal to atypical to cancerous very quickly, which wouldn’t give researchers much time to disrupt this process. If my cancer grows slowly, surveillance is more likely to help, because there’s a longer time window in which treatment can stamp it out. Of course, no one knows quite how high their flame is—how fast their potential cancer might grow—in advance.
In her book Preventive Strikes, Ilana Löwy, a senior researcher at the French National Institute of Health and Medical Research, discusses the practice of routine screening for another disease—cervical cancer—and points out that “one of the rarely discussed drawbacks of such screening is irreversibly generation of uncertainty…Diagnosed with a potentially threatening condition with an uncertain meaning, they [the patients] are not sure if they should see themselves as sick or healthy.”
As a woman positive for a BRCA mutation, I bear this uncertainty doubly, both because I am frequently screened for cancer and am therefore more likely to receive ambiguous results, but also because the BRCA test itself is a sort of screening for pre-cancer. I may not have any precancerous lesions inside me, but I have been told that I have a potentially life-threatening mutation inside every cell of my body. After my genetic results came back, I no longer felt like the physically healthy twenty-seven-year-old newlywed that I was. Instead I became someone who went to the doctor more than ten times a year, like a good patient, to make sure I wasn’t sick yet. I lived in a state of betweenness, in a no-man’s-land straddling the worlds of sick and healthy.
Most of the studies done have looked at screening in the general population and not in women at high risk of developing breast cancer. Truly, I’d like to see some studies performed on my risk group to determine the basic efficacy of ovarian and breast surveillance in preventing cancer death. I shouldn’t hold my breath, though. Large studies are expensive to produce, weighed against the comparatively small number of BRCA carriers who would benefit, and they require lots of patients. Are there even enough BRCA women who would be willing to be randomly assigned to the screening or nonscreening arm of such a trial? Would such a study even be ethical? I guess living with the flawed human screening we have now, and living with the uncertainty generated, is all part of what it means to be a mutant.